Quagga mussels eradicated from lake with low doses of ionic copper
David Hammond
Earth Science Laboratories, Inc.
903 N 47th Street, Suite 105
Rogers, AR 72756
Gavin Ferris
SOLitude Lake Management, Inc.
PO Box 969
Virginia Beach, Virginia 23451
This article originally appeared in Management of Biological Invasions (2019) Volume 10, Issue 3: 500–515. A PDF version can be downloaded here.
Introduction
Zebra mussels (Dreissena polymorpha Pallas, 1771) and quagga mussels (D. rostriformis bugensis Andrusov, 1898), known collectively as dreissenid mussels, have established themselves as nuisance aquatic invasive species throughout many of the major watersheds of North America. The resulting environmental and economic damage have been extreme, earning them recognition among the continent’s most damaging aquatic invasive species (IUCN 2018; Western Governors’ Association 2018; Fetini 2010). Native to the Caspian Sea region of Eastern Europe, dreissenids were first detected in North America in 1985 in Lake St Clair (Claudi and Mackie 1994), which is located between Lake Huron and Lake Erie. In the subsequent 5 years they extirpated 12 species of native mussels by physically smothering and out-competing them for food (Nalepa et al. 1996). Other native mollusks also suffered massive reductions in range and population (Nalepa et al. 1996).
Economic impacts from invasive dreissenid mussels have been particularly severe in water treatment and power generation facilities, where prompt and effective protection against biofouling is often essential. Estimates of the economic impacts from invasive mussels vary widely, with several sources citing costs to the Great Lakes region in the range of US$3 billion to $7 billion over a 10-year span (Cataldo 2001; Sun 1994; Lovell et al. 2006) and a mean of US$204,000 per facility in a 7-year period 1989–1995 (O’Neill 1997). The National Center for Environmental Economics estimated annual costs per facility for mussel control averaged US$83,000 for hydroelectric plants, $143,000 for fossil fuel plants, and $833,000 for nuclear power plants (Lovell and Stone 2005). Chakraborti et al. (2016) reported that the operations and maintenance costs alone for mussel control in drinking water plants varied from US$3.39 to $9.07 per megaliter treated for small facilities and from $0.22 to $3.34 per megaliter treated for large facilities. A survey of water treatment facilities found that 37% had mussels present in their infrastructure and annual expenditures per facility averaged $44,000 in the initial years of infestation and $30,000 after control procedures had become optimized (Connelly et al. 2007).
Other lake-related activities also suffer impacts from zebra and quagga mussel infestations including lost time and costly labor for roadside boat inspection stations, boat owners needing to clean off their craft and docks, decreased access and usability of shoreline areas due to the sharp shells, and impacts that have proven difficult to quantify regarding fisheries and potential degradation of natural spawning grounds (O’Neill 1997). The economic losses from interference in recreational activities are difficult to estimate but are also considered significant. The Pacific Northwest’s Columbia River Basin is the last major watershed in the continent still free of dreissenids; in 2016, the Pacific Northwest Economic Region Foundation reported that current costs associated with prevention such as watercraft inspection, outreach, and monitoring in that and adjoining regions exceeds US$13 million annually, but that the estimated cost of dreissenids becoming established would be more than $500,000,000 per year (Pacific Northwest Economic Region Foundation and Pacific States Marine Fisheries Commission 2015).
The need for viable and cost effective dreissenid mussel control agents is therefore acute. Hundreds of lakes across the continental U.S. are already infested, yet hundreds of thousands remain uncolonized so both preventative and control measures are needed to manage the spread and containment of dreissenid mussels. Once invasive mussels are widely distributed throughout a large lake—for example greater than 400 hectares—there is little hope for eradication and control efforts must focus on management. But for Rapid Response efforts in recently-infested lakes and for lakes less than about 200 hectares, there is a growing body of evidence, cited below, that effective control and even complete eradication can be both feasible and cost effective (Lund et al. 2017; Fieldseth and Sweet 2016).
In 2006, zebra mussels were successfully eradicated from a 5-hectare lake in Virginia—the first reported instance of eradication of invasive mussels from an open water body—using a dose of approximately 100 mg/L potassium as potash (unrefined potassium chloride) at a contract cost of US$365,069, equivalent to approximately $0.54 per cubic meter treated (Fernald and Watson 2014).
Conventional copper sulfate (granular as CuSO4·5H2O) was used to treat for invasive mussels in a Nebraska lake by applying 1.0 mg/L to the entire water body in two separate treatments, once in September 2008 and again in April 2009. Subsequent monitoring indicated the treatment was effective but the effort was marred by the death of 21 different species of fish during the 40 days following each treatment, including 17,500 kg in 2008 and 1,400 kg in 2009 (Offutt Air Force Base 2009), and also because mussels were again detected in the lake in 2014. It is not known whether those mussels were survivors or were reintroduced, but the project contributed to a perception that treatment with copper poses substantial risk of high mortality rates among non-target fish.
A study using quagga mussels collected at Lake Mead, NV in 2010–11 demonstrated that an ionic copper product called EarthTec QZ is effective against both adults and veligers and proposed its potential for preventing further colonization by this invasive species (Watters et al. 2012). Other studies have shown that copper is effective against veliger and pre-veliger life stages at dose rates at least an order of magnitude lower than needed to kill adults (Kennedy et al. 2006; McCartney 2016), which suggests a method for interrupting the reproductive cycle of the pest while causing minimal impacts on non-target organisms.
A comparative study commissioned in 2012 by California Department of Water Resources measured efficacy of four different copper products applied to control zebra and quagga mussels in 96-hour mesocosm bioassays. EarthTec QZ yielded 100% mortality of zebra mussels whereas copper sulfate pentahydrate and two other liquid copper products produced only 25–35% mortality, even though all four were dosed at the same active ingredient concentration of elemental copper (Claudi et al. 2014). This study illustrated that molluscicidal efficacy of copper products is dependent not only on the concentration of active ingredient, but also how it is formulated.
Between 2014 and 2017, five lakes in Minnesota were treated with EarthTec® QZ during Rapid Response efforts supervised by the Minnesota Department of Natural Resources (Cattoor 2018; Hammond 2017). Barriers were erected around the infested areas and treatment was begun within 20–40 days of the discovery of mussels. In four of the five lakes local agencies reported that the treatments had successfully eradicated mussels within the treatment areas, and in the fifth there was 70% mortality before snow and ice prevented further treatments (Keegan Lund and Justin Valenty, personal communication). Effects on fish and other non-target organisms were not quantified, but none were readily visible. In Ruth Lake, a cluster of native mussels was killed by the treatment, but no fish were killed and 3 years later no zebra mussel adults or veligers are being detected and native mollusks are thriving. In three of the five lakes, mussels were already known or subsequently found to be present outside the treatment area, undermining hopes of preventing lake-wide colonization. One of the lessons from these partial-lake Rapid Response treatments was to ensure the treatment area is sufficiently large to include mussels that might have escaped population surveys (Lund et al. 2017).
EarthTec QZ is currently used to treat water intakes and pipelines of municipal water treatment plants for prevention of dreissenid mussel colonization and infrastructure biofouling, where a dose of 1 ppm (equivalent to 0.06 mg/L as copper) has proven effective (Claudi et al. 2016). It is NSF-certified for use in drinking water (http://info.nsf.org/Certified/Pws Chemicals/Listings.asp?Company=14860&Standard=060) and is one of the few products approved by Environmental Protection Agency (EPA) for use to control invasive mussels in open waters.
This report describes the use of the acid-stabilized ionic copper formulation called EarthTec QZ to treat an entire lake heavily infested with quagga mussels for the purpose of eradicating the invasive species. This is the first recorded instance of a lake-wide eradication effort of quagga mussels and is also the largest and deepest lake known to have been the subject of an effort to completely eradicate either quagga or zebra mussels.
Materials and methods
Site Characteristics
Billmeyer Quarry (Figure 1) is located on property owned by the Lancaster County Solid Waste Management Authority in Conoy Township, Pennsylvania, 30 kilometers southeast of the city of Harrisburg, PA (Figure 2). Under average conditions the lake has a surface area of 12 hectares, a maximum depth of about 35 m in the west basin, 27 m in the east basin, and a total volume calculated at 1.84 million cubic meters. Ice sometimes forms on Billmeyer Quarry for short periods in winter and the water is thermally stratified during summer months with the epilimnion averaging 0–5 m, metalimnion 5–10 m, and hypolimnion > 10 m depth. The lake has a rocky substrate and normally has no surface connection to other water bodies. The Susquehanna River passes 200 m to the southwest at 7 m lower elevation and is known to be infested with zebra mussels but quagga mussels have never been reported or confirmed in the river. Quagga mussels were first confirmed in the Billmeyer Quarry in 2005 and at the time of this treatment in fall of 2017 they were present throughout. Several dives in the top 6 meters to informally survey the mussel population suggested that the density of adult mussels was highest in the top 3 meters.

Figure 1. Aerial view of Billmeyer Quarry, with the Susquehanna River to the southwest. The west basin (left) is the larger and deeper of the two (photo from Google Earth).
Figure 2. Figure 2. Approximate location of Billmeyer Quarry.
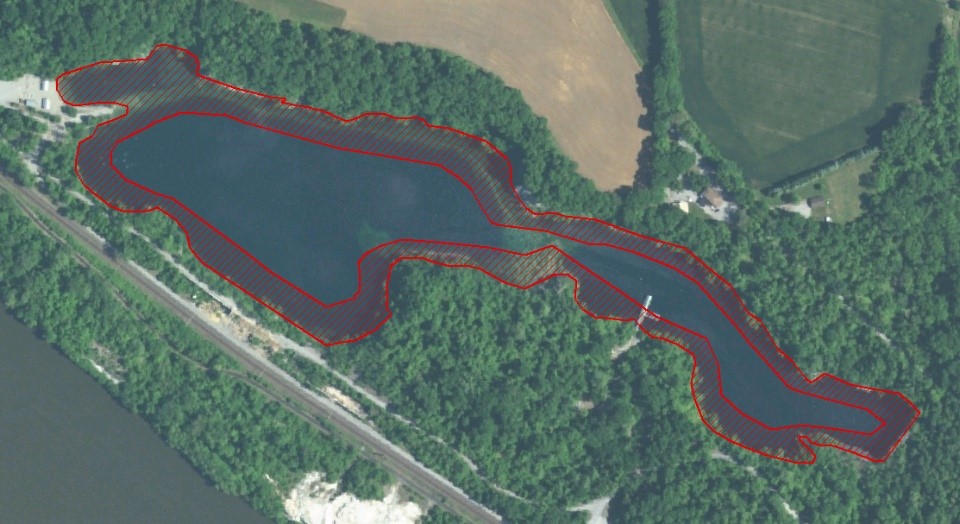
Figure 3. Treatment zone, comprising 50% of the surface area.
Product and Application
EarthTec QZ is a translucent blue colored liquid with 5% copper by weight, a viscosity of 1.88 centipoises – which is similar to water, and a specific gravity of 1.188. It is registered with the Pennsylvania Department of Environmental Protection (DEP), the treatment in Billmeyer Quarry followed label directions, and was authorized under a standard permit.
The treatment protocol targeted a copper concentration of 0.2 mg/L as measured within the treatment area, to be maintained at least until all caged mussels were dead. Product was applied to 50% of the lake’s surface acreage around the perimeter (Figure 3) and allowed to disperse towards the center without mechanical assistance. Product was introduced from a boat with a 5 hp Honda GP15 pump. Polyethylene tubing weighted at the discharge end with downrigger weights was used to distribute it equally among the targeted depths. Treatments at shallower depths were applied from the powered boat; deeper treatments were applied while drifting to reduce the likelihood of hoses tangling with submersed timber and other debris. Dissolved copper was monitored within the treatment area and when copper concentrations dropped below 0.1 mg/L, the dose of product needed to theoretically reestablish a concentration of 0.2 mg/L as copper was calculated and re-applied.
The first treatment occurred Sept 5, 2017 and used 6,245 liters of EarthTec QZ applied to 50% of the lake’s surface, with the majority dispensed at 2 m, 6 m, and 9 m. The second treatment occurred on Day 7 and consisted of 4,164 liters applied at depths of 2 m, 6 m, 18 m, 21 m, and 26 m. The third and final treatment occurred on Day 37, when 3,123 liters were applied targeting a depth of 9–12 m in response to low measured copper concentrations and slow mortality rates observed in the metalimnion. In sum, three treatments were applied over a total period of 37 days, for a cumulative total of 13,531 liters of EarthTec QZ dosed into 1.84 million cubic meters of lake volume, equivalent to 0.44 mg/L as ionic copper.

Figure 4. Example of a cage stocked with 20 adult mussels and ready for deployment.
Operating from a boat, grab samples of water near the mussel-containing cages were collected using a Kemmerer sampling tube and analyzed immediately for residual copper concentration as a way to monitor the animals’ exposure to the active ingredient. Copper concentrations were determined by means of the porphyrin test method (Method 8143, https:// www.hach.com/asset-get.download.jsa?id=7639983668), the bicinchoninate test method (Method 8506, https://www.hach.com/asset-get.download.jsa? id=7639983601), and a DR900 spectrophotometer, all from Hach Chemical Co (Denver, CO). Samples were collected from cage locations at different depths to give a sense of copper concentrations across all thermal strata of the lake.
Biology
Mortality of mussels was monitored using adult mussels collected from the lake and placed in cages (n = 48) for easy observation. Cages were fabricated as mesh containment bags using tule fabric, then suspended inside 6” lengths of clear polycarbonate pipe measuring 4” in diameter (Figure 4). Mussels were hand collected from the quarry. Dead and damaged mussels were discarded, retaining only the most robust individuals as test subjects. These mussels were then partitioned 20 per cage, with each cage housing the full variety of size classes available, from 6 to 25 mm. No additional acclimation steps were deemed necessary.
Adult mussels were considered dead if the shell was gaping and the flesh completely absent or noticeably decomposing. If the shell was closed or slightly open yet unresponsive, the individual was scored as alive and carefully replaced in the cage. The cages were suspended at 12 pre-selected locations and 4 depths per location throughout the lake with a minimum depth of 3 m and a maximum depth of 100 m. Nine of the cage locations were distributed around the periphery of the lake and three in the center.
Veliger tows used for detection of environmental DNA (eDNA) were conducted using a 64-micron mesh plankton tow net with a 13-cm diameter opening (Aquatic Research Instruments, Hope, ID). The volume collected was approximately 1,000 liters, once during the first week of December 2017 and again on July 4, 2018. The analysis was performed by specialists at the Montana Conservation Genomics Lab, a cooperative lab focusing on eDNA and conservation genomics run by the Flathead Lake Biological Station and the W.A. Franke College of Forestry and Conservation at the University of Montana.
eDNA was extracted from plankton tow net samples and tested for aquatic invasive zebra and quagga mussel DNA (Dreissena polymorpha and Dreissena rostriformis bugensis) using a genus‐specific TaqMan quantitative PCR (qPCR) assay targeting a region of the dreissenid mussel 16S gene. All sample handling, storage, and DNA extractions were conducted in a separate facility designated only for eDNA samples to prevent possible false positives from contamination of the extractions by PCR amplification products or other potential sources of mussel DNA.
Invasive mussel amplification was noted for any independent qPCR reaction yielding more than 10 copies of dreissenid DNA per μl in a sample. Initially, three or more independent qPCR reactions were conducted per sample. Any sample with a positive qPCR reaction in the initial set of three replicates was re‐tested, re‐extracted and six additional independent qPCR reactions using a second extraction were run to verify the results. A plankton tow sample was considered to have a positive test result if all 12 independent qPCR reactions from both DNA extractions displayed amplification above 10 copies of target DNA per μl threshold.
If a sample did not exceed the positive amplification threshold, but dreissenid DNA in concentrations above one copy per μl (the technical minimum concentration of detection) was detected and the reaction produced a clear sigmoidal amplification curve, the sample was also re‐tested in an effort to achieve results meeting our amplification thresholds. If inconsistent low level amplification (below 10 copies/μl) was also observed in the second round of testing (e.g., in one or more of six additional independent qPCRs), test results were inconclusive and the sample was noted as having “Low/Variable Amplification”. Test results for samples with low/variable amplification are considered disputable, failed to meet the stringent criteria for positive amplification, and are NOT positive samples. Inconclusive samples with low/variable amplification require additional sampling and/or testing to produce definitive results.
An Internal Positive Control (IPC) assay was used to differentiate true target (e.g. dreissenid mussels) qPCR negatives, where no dreissenid DNA is present, from false qPCR negatives where a small amount of dreissenid DNA is present but does not amplify due to PCR inhibitors. In a sample, if amplification of the IPC DNA is delayed more than 5 PCR cycles than observed in the control, that sample is diluted and re‐tested.
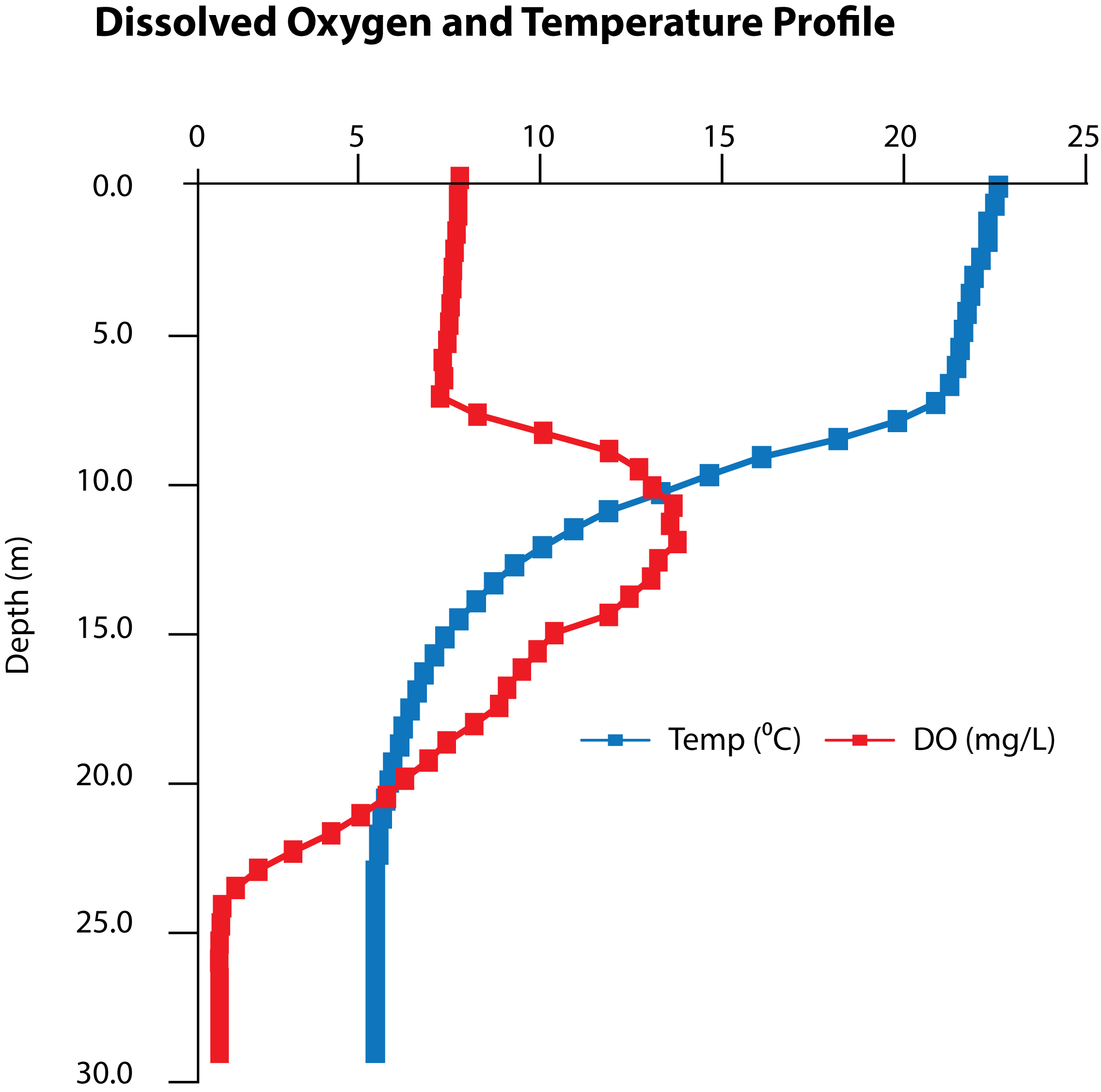
Figure 5. Temperature and dissolved oxygen profiles vs depth as measured at location Y, near the center of the lake’s west basin on 10/10/2017, Day 35.
Effects on non-target organisms were assessed by observation of fish during and after treatments, and by plankton sampling one year following treatment. Because EarthTec QZ is a liquid formulation and highly soluble in water, it was assumed that impacts would be concentrated in the water column rather than in the benthic zone. Plankton sampling was initiated and completed the morning of September 27, 2018, one year after treatment, in the west (main) basin. The quarry was accessed using an inflatable raft with oars to reach four sampling sites. Sampling was conducted using a 0.5 m diameter plankton net with 63 micron mesh. Vertical tows were taken from the bottom to the surface. The estimated volume sampled at each site was 2872, 4188, 3889 and 4608 liters. The samples were reduced down to 40 mL and the number of organisms present was estimated by using a Nikon model 78788 microscope, with cross polarization filter added to aid in identification of mollusks. Individuals were counted in no fewer than four random fields of view, each measuring 19.6 mm2, and the results were averaged.
Results
Water temperature during the treatment period ranged widely, from a high of 23 °C near the surface to < 5.5 °C near the bottom. Dissolved oxygen concentrations measured 10 mg/L near the surface, increased to a maximum of 15 mg/L at 12 m, and then declined at greater depths to a minimum of < 0.5 mg/L (Figure 5). The observed temperature and D.O. profiles matched historical measurements at the lake that indicated there could be sufficient D.O. (about 5 mg/L) to support mussels at a depth of at least 24 m. Treatment techniques therefore sought to ensure a lethal concentration of product reached that depth.
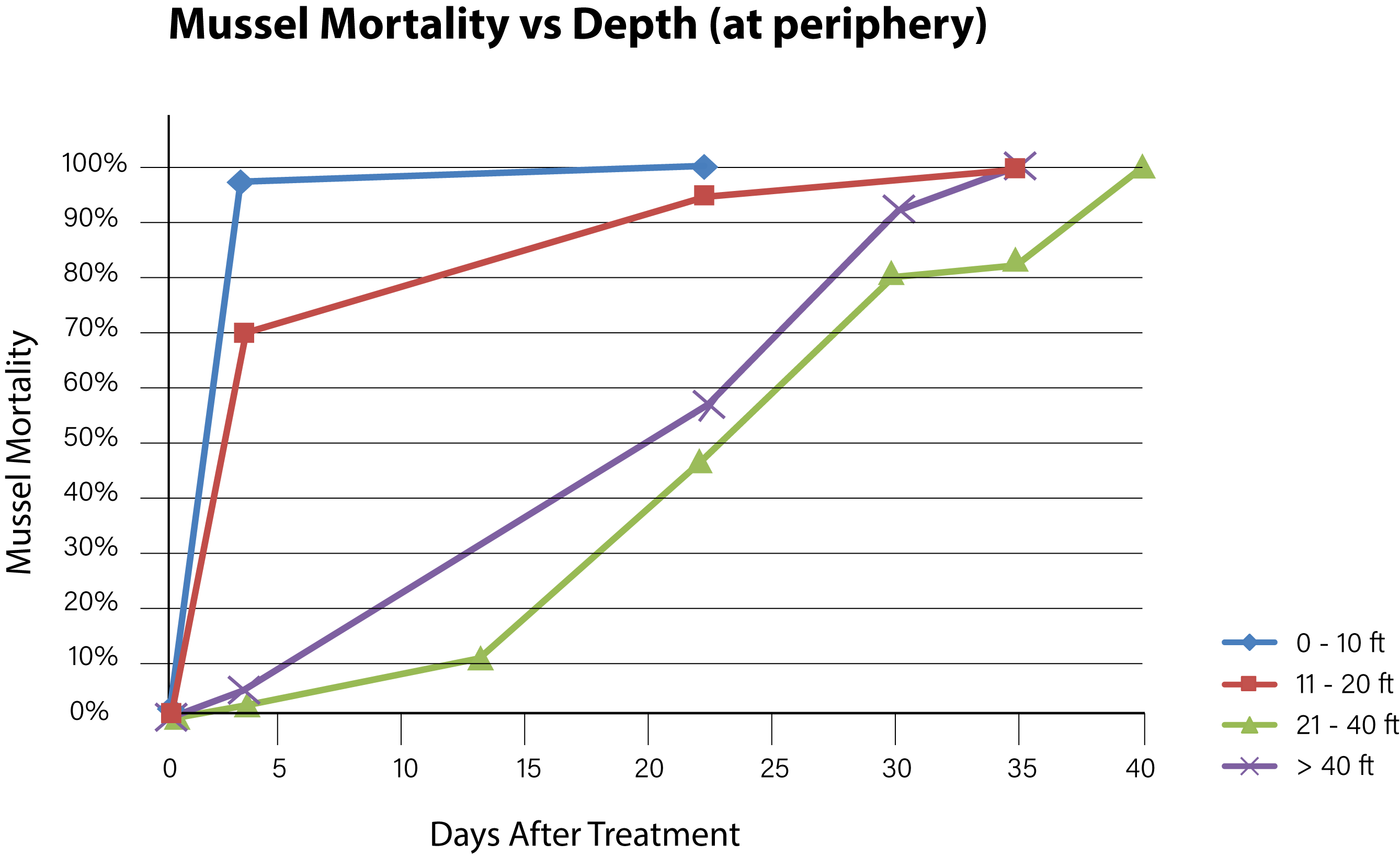
Figure 6. Time needed to achieve 100% mussel mortality at various depths along the lake periphery.
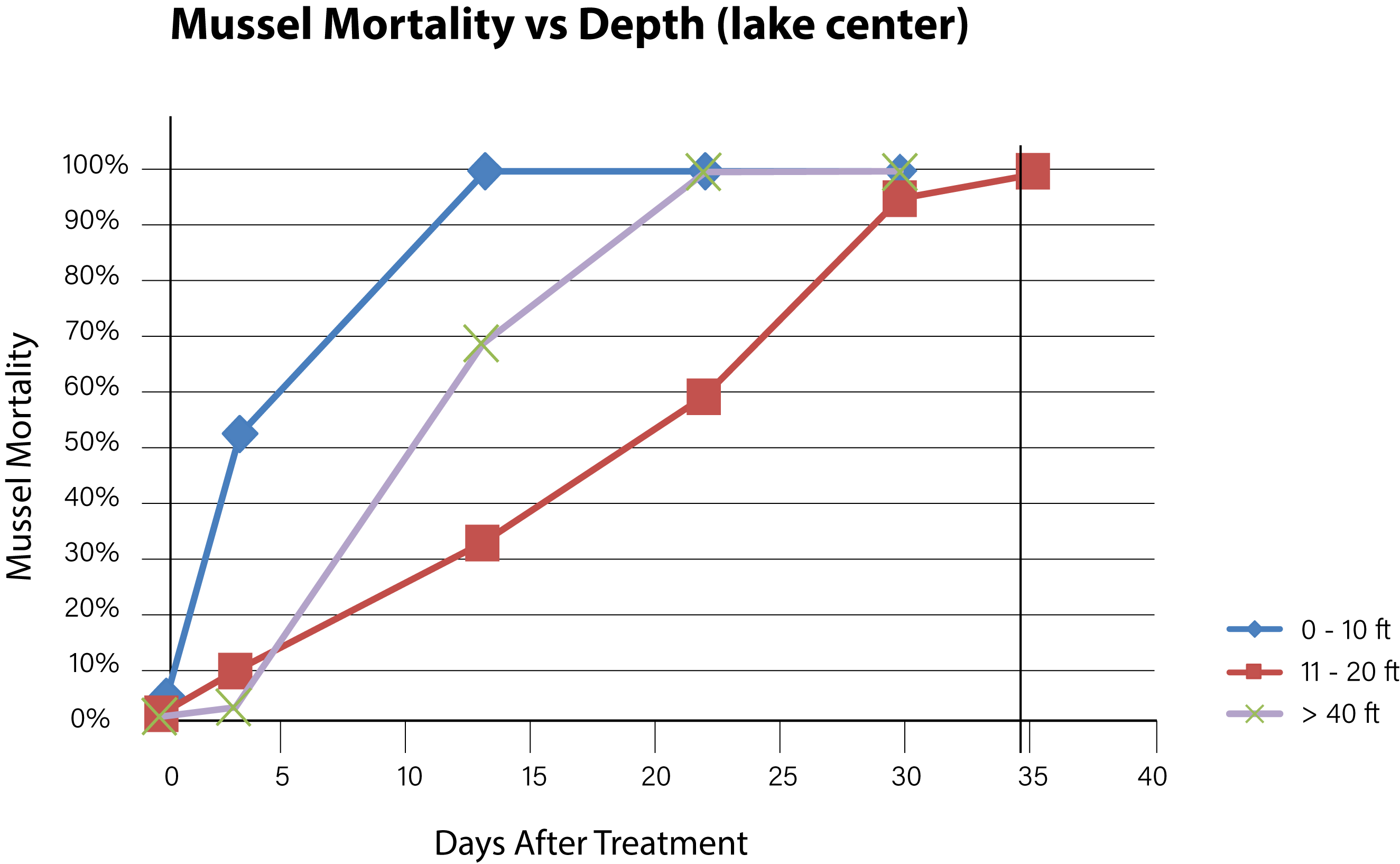
Figure 7. Time needed to achieve 100% mussel mortality at various depths in the lake center.
Background concentrations of copper prior to the first treatment were measured to be from 1.0 to 1.5 ug/L as dissolved copper. Alkalinity of the lake water pre-treatment was confirmed at > 200 mg/L and hardness at 280 mg/L. Adult mussels began to die within 3 days of the first treatment with EarthTec QZ and mortality was fastest among mussels located in the top 6 m of the water body, where the water was warmest and well mixed. The mean exposure time required for 100% mortality of caged mussels in the top 6 m of water was 10 days (n = 28, standard deviation = 11.1). Mortality of caged mussels at greater depths continued to increase with length of exposure, following a typical dose-mortality relationship as illustrated by a curve with sigmoidal shape (Figures 6 and 7). Due to the high concentration of mussel population in the first 6 m of water and based on the high mortality observed in that zone, it was estimated that more than 90% of the lake’s mussel population was dead at 22 days post-treatment. The death of the last caged mussel was confirmed 40 days after the initiation of treatment, in a cage that had been placed at a depth of 9 m below the surface. There was little difference between mortality observed in the periphery locations and those at the center-of-lake. The mussels that survived the longest were those in cages positioned within the metalimnion located within the 6–12 m depth category and had a mean time to total mortality of 33 days (n = 7 standard deviation = 7.0). Mussels placed in the hypolimnion (> 12 m) had a mean time to total mortality of 24 days (n = 12, standard deviation = 9.7).
Copper concentrations were measured at multiple depths several times throughout the treatment period. The results of these measurements guided “bump” treatments intended to boost copper concentrations in the metalimnion and hypolimnion. Copper concentrations remained low in the metalimnion throughout the observed period, with the maximum recorded concentration of 0.17 mg/L. By contrast the epilimnion and hypolimnion had maximum recorded copper concentrations of 0.88 mg/L and 0.51 mg/L, respectively (Figures 9 and 10).Visual inspection of the shoreline after partial pump-down of the quarry in early November 2017 (Figure 8) revealed shoreline areas densely littered with empty mussel shells; no live mussels were found. Plankton samples collected post-treatment on December 6 at depths from 0 to 12 m contained no live veligers although one empty veliger shell was found.
Two environmental DNA samples (eDNA) were collected using plankton tows at two locations in the quarry (east and west), one set the first week of December 2017, and one set the first week of July 2018 when any surviving mussels were expected to have had a chance to spawn. The east December sample amplified in only one of six replicates and produced a sigmoidal amplification curve (low/variable amplification) of very low eDNA concentrations. The west December sample consistently amplified below the lowest threshold of detection and produced sigmoidal amplification curves in all replicates. This suggests that dreissenid DNA may have been present in December 2017 in very low levels below the qPCR detection limit used to establish a definite positive. Both east and west samples collected in July 2018 were negative for dreissenid mussel DNA.
Due to the depth of this lake an accurate estimate of the total fish population was not practical. Nonetheless, large numbers of fish were observed before, throughout, and after the treatment period. Species observed included largemouth bass (Micropterus salmoides), smallmouth bass (Micropterus dolomieu), gizzard shad (Dorosoma cepedianum), bluegill sunfish (Lepomis macrochirus), channel catfish (Ictalurus punctatus), and black crappie (Pomoxis nigromaculatus). Ten dead gizzard shad were observed floating 24 hours after the second application of product.
The results of the plankton sampling conducted one year after treatment are summarized in Table 1. In addition to the zooplankton quantified in Table 1, aquatic life representing higher trophic levels were also observed to be present in the plankton tows, including caddisfly larvae, mayfly nymphs, phantom midges, Dytiscid larvae, Haliplid larvae, five live mollusks (clams), and rotifers too numerous to count, all of which suggests the base of the food web remains robustly intact. While the samples collected were described by impartial analysts as qualitatively “very rich” in zooplankton numbers and diversity, it is unknown how these numbers compare to pre-treatment populations.

Figure 8. Upon pump-down of the lake, the empty shells of dead mussels were found along the newly-exposed shoreline (photo courtesy of SRBC).
Discussion
The purpose of treating Billmeyer Quarry was to minimize the possibility of transferring quagga mussels that might get entrained in the pumped water during a planned pumping event designed to supplement flows in the adjacent Susquehanna River as part of a consumptive mitigation initiative. Stakeholders reasoned that achieving complete eradication of mussels from the quarry would be a valuable secondary objective and would make it easier to undertake future pumping events. Consequently, this project was conceived not as a research study but as a practical management measure.
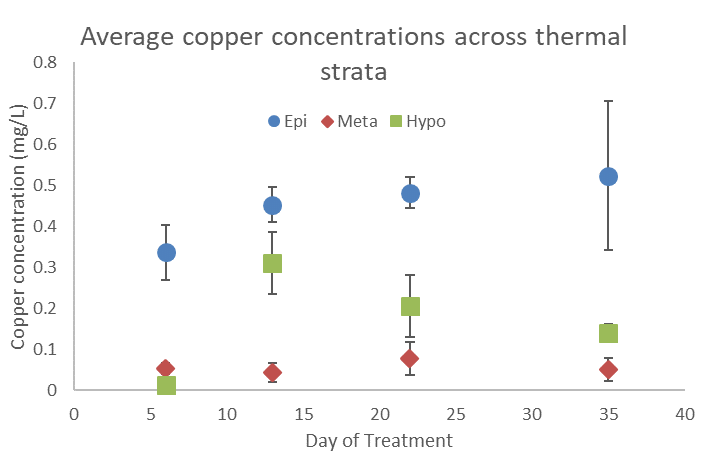
Figure 9. Average copper concentrations across thermal strata throughout the treatment period. Error bars represent 1 standard error.
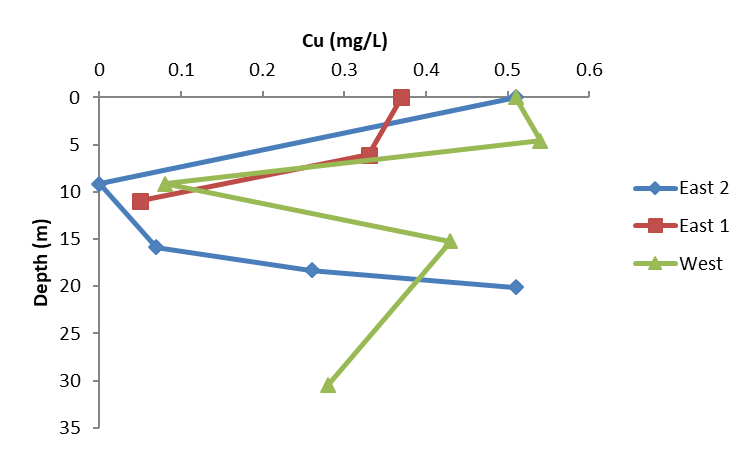
Figure 10. Copper concentrations as measured in grab samples taken at different depths and locations within the center of the lake’s two basins on Day 13, Sept 18, 2017. A thermally isolated layer of water near 9–15 m deep resisted mixing and the majority of samples taken there measured less than 0.05 mg/L as copper.
| Table 1. Enumeration of zooplankton abundance (average number of organisms/L) at one year post-treatment, in Sept 2018. | ||||
| Site 1 | Site 2 | Site 3 | Site 4 | |
| Cladocerans | 25 | 17 | 16 | 15 |
| Copepods | 3 | 2 | 2 | 2 |
| Ostracods | 3 | 3 | 3 | 3 |
| Nauplei | 2 | |||
The treatment accomplished the primary objective of minimizing risk of mussel transfer during the pumping event. Regarding the secondary objective, it is difficult to prove eradication but no live mussels have been found and eDNA tests have been negative, so the level of control achieved was remarkable, particularly considering the low dose of chemical applied.
In any population, there are natural variations in the individuals’ sensitivity to toxicants, so exposure to environmental pressures or control measures will cause some to die quickly and others to survive significantly longer. This principle was illustrated by the fact that mussels began to die within 4 days of the initial product application, with percent mortality increasing over time; 90% of the caged test mussels were dead at 22 days of exposure. The last caged mussels survived for 40 days.
The lake’s thermocline also played a role in ease of treatment and rate of mussel mortality by making it difficult to maintain an effective concentration of copper in the metalimnion. Biological and physicochemical data collected during the project period were similar to those observed in previous years, revealing a pronounced thermocline at 6–12 m depth.
In the week following treatment, measurements of dissolved copper concentrations in grab samples taken at different locations and depths suggested that water above and below the thermocline was mixing well, while copper concentrations within the metalimnion itself remained markedly lower, ranging from < 0.01 mg/L to 0.08 mg/L (Figure 10). It is possible that a phytoplankton bloom present around the thermocline—suggested by the positive heterograde dissolved oxygen profile (Figure 4)—was incorporating into its biomass whatever dissolved copper reached that depth. Another possible explanation is that since the EarthTec QZ used for treatment was stored in a nearby warehouse with ambient temperatures that at times exceeded 40 °C, the temperature difference between the treatment plume and the water could have discouraged vertical mixing, especially penetration of and below the thermocline. Given the difficulty encountered in achieving and maintaining lethal copper concentrations in the metalimnion, one consideration for future projects in seasonally stratified lakes could be to wait until turnover occurs.
If future sampling confirms that this eradication effort was successful, it will be the first recorded instance of eradicating quagga mussels from an entire lake. EarthTec QZ was used to eradicate zebra mussels from small lakes in two previous cases: a private lake in Indiana and a lake in the Minnesota Zoo (unpublished data). The Billmeyer Quarry, at 12 hectares and 35 m deep, is the largest and deepest lake to be the subject of a lake-wide effort to eradicate quagga mussels.
The total cost of the RFP was US$109,400. To provide some context, this is about 30% of the cost required to treat Millbrook Quarry in Virginia in 2005 with potash—a water body less than half the size and volume of Billmeyer Quarry—and approximately one-ninth the cost when compared on a per-volume basis. The total project cost for treatment of Billmeyer Quarry worked out to approximately US$0.06 per cubic meter treated, with chemical cost comprising slightly less than half of that.
The total amount of copper applied throughout the course of treatment was equivalent to 0.44 mg/L, which is noteworthy because the EPA-labeled rate for copper-based algaecides allows up to 1.0 mg/L in a single treatment event.
Non-Target Organisms
Efforts to control invasive mollusks must consider the collateral impact to native mussels, especially in areas where freshwater mussel species may be threatened or endangered. Billmeyer Quarry is an artificially created waterbody not directly connected to any other waterbody and has no naturally occurring mussel populations. Where native mussels are present, temporary relocation and/or restocking of natives after the water body has been reclaimed are mitigation options that should be considered.
Since invasive mussels are known to physically smother small animals and may degrade fish spawning grounds, effects on non-target organisms should be evaluated in the context of potential to reclaim the treated water body. The main drivers for this project were related to management of the water itself and quantification of impacts on non-targets was not a central objective, but those impacts appear to have been minor.
The fact that only one fish species, gizzard shad, was observed to experience an adverse effect suggests that this species may be more sensitive to copper than other fish present in the quarry. Still, many other individuals of this species were observed in apparently good health in other parts of the lake, and no other fish died during the remainder of the treatment and observation period, so we hypothesize that these few fish were probably trapped during the application in an area that happened to receive a higher-than-average concentration and were unable to swim out of the treatment plume.
No other adverse effects to non-target macrofauna species were observed during the treatment and observation period.
Conclusions
All the caged mussels died within 40 days of the start of treatment on September 5, 2017. Inspection of the shoreline in the subsequent months when water levels were lower revealed only the empty shells of dead mussels. Plankton tow sampling for environmental DNA (eDNA) samples was conducted at an east and west location in the quarry, once in the first week of December 2017, and again the first week of July 2018 when any surviving mussels were expected to have had a chance to spawn. The eDNA results for the December samples suggest that dreissenid DNA may have been present in very low levels below the qPCR detection limit used to establish a definite positive. Both samples in July were negative for dreissenid mussel DNA. Note that interpretation of sample test results pertain only to the absence or presence of dreissenid mussel DNA in the samples. Negative results do not definitively establish that zebra and quagga mussels are absent from a given area or waterbody (even from which all samples were negative). While it is virtually impossible to know with absolute certainty whether an eradication effort has been completely effective, the eDNA data are concordant with the other tests suggesting strong suppression or eradication of mussels by the treatment.
The total cumulative amount of EarthTec QZ applied for this eradication effort is equivalent to 0.44 mg/L as copper, which represents less than half the dose of copper that EPA allows (1.0 mg/L) in a single application for molluscicidal or algaecidal purposes. The fish population remained stable throughout the treatment period and thereafter. This project demonstrates that low doses of acid-stabilized ionic copper offer an effective and economical means of control of invasive dreissenid species in open waters.
Acknowledgements
Treatment of Billmeyer Quarry to accomplish control of quagga mussels was paid for by the Susquahanna River Basin Commission. The treatment protocol was designed and supervised by Earth Science Laboratories, Inc., and executed by SOLitude Lake Management, Inc. Plankton sampling and analysis was performed by Normandeau Associates, Drumore, PA. Analysis of eDNA was performed by Montana Conservation Genomics Lab, Polson, MT. The authors would like to express particular gratitude and thanks to: David Carrington, Ed Shimer, Mark Bellaud, Bo Burns, Keith Gazaille, Bob Schindler, Jason Emmel, Vic Dicenzo, Chris Doyle, Todd Schramm, Vincent Giordano, Susquehanna River Basin Commission, Pierre MaCoy, Ellyn Campbell, Lancaster County Solid Waste Authority, Mark Reider, Dan Brown, Billy Graham Jr., Normandeau and Associates, Doug Royer, Steve Kauffman, Steve Adams, Dulaney Miller, Stephen Amish, RNT Consulting, Renata Claudi, and Tom Prescott, PE. They authors would also like to acknowledge the thorough review and thoughtful suggestions provided by the team of reviewers and editors.
References
Cataldo R (2001) Musseling in on the Ninth District Economy. Fedgazette 13(1): 15–17.
Cattoor K (2018) Zebra Mussel Pilot Projects: a discussion of the recent eradication efforts in Minnesota and what next? Upper Midwest Invasive Species Conference, October 16th, 2018, Rochester, Minnesota.
Chakraborti RK, Madon S, Kaur J (2016) Costs for Controlling Dreissenid Mussels Affecting Drinking Water Infrastructure: Case Studies. Journal AWWA 108: E442–E453.
Claudi R, Mackie GL (1994) Practical manual for zebra mussel monitoring and control. Lewis Publishers, CRC Press, Boca Raton, Florida, 227 pp.
Claudi R, Prescott TH, Mastisky S, Coffey H (2014) Efficacy of Copper Based Algaecides for Control of Quagga and Zebra Mussels, Report for California Department of Water Resources, Aquatic Nuisance Species Program, 58 pp.
Claudi R, Schaffer R, Hammond D, Prescott TH (2016) Efficacy of EarthTec QZ for Control of Dreissenid Mussels, report for RNT Consulting, Picton, Ontario, Canada, pp 1–10.
Connelly NA, O’Neill CR Jr, Knuth BA, Brown TL (2007) Economic impacts of zebra mussels on drinking water treatment and electric power generation facilities. Environmental Management 40: 105–112.
Fernald RT, Watson BT (2014) Eradication of zebra mussels (Dreissena polymorpha) from Millbrook Quarry Virginia: rapid response in the real world. In: Nalepa TF, Schloesser DW (eds), Quagga and zebra mussels: biology, impacts and control. CRC Press: Boca Raton, FL, pp 195–213.
Fetini A (2010) Zebra Mussels: They’re Taking Over; Top 10 Invasive Species. Time Magazine Feb. 02, 2010.
Fieldseth E, Sweet J (2016) Rapid Response to Zebra Mussel Infestation, Lake Minnewashta, Carver County, MN. Report by the Minnehaha Creek Watershed District, Minnetonka, MN, pp 1–24.
Hammond D (2017) EarthTec QZ: Control of dreissenid mussels with a more rational use of copper. Conference Proceedings, 20th International Conference on Aquatic Invasive Species, October 22-26, 2017, Fort Lauderdale, FL, USA.
IUCN (2018) International Union for Conservation of Nature. “100 of the World’s Worst Invasive Alien Species,” Global Invasive Species Database, Invasive Species Specialist Group (accessed 23 October 2018).
Lovell SJ, Stone SF (2005) The Economic Impacts of Aquatic Invasive Species: A Review of the Literature. EPA Working Paper #05-02
Lovell SJ, Stone S, Fernandez L (2006) The Economic Impacts of Aquatic Invasive Species: A Review of the Literature. Agricultural and Resource Economics Review 35: 195–208.
Lund K, Bloodsworth Cattoor K, Fieldseth E, Sweet J, McCartney MA (2017) Zebra mussel (Dreissena polymorpha) eradication efforts in Christmas Lake, Minnesota. Lake and Reservoir Management 34: 7–20.
Kennedy AJ, Millward RN, Steevens JA, Lynn JW, Perry KD (2006) Relative Sensitivity of Zebra Mussel (Dreissena polymorpha) Life-stages to Two Copper Sources. Journal of Great Lakes Research 32: 596–606.
McCartney MA (2016) Summary Report: Field evaluation of toxicity of low-dose molluscicide treatments for zebra mussel veliger larvae-potential applications in lake management.
Prepared for Minnehaha Creek Watershed District, Minnesota. Dec 21, 2016, pp 1–52
Nalepa TF, Hartson DJ, Gostenik GW, Fanslow DL, Lang GA (1996) Changes in the freshwater mussel community of Lake St. Clair: from Unionidae to Dreissena polymorpha in eight years. Journal of Great Lakes Research 22: 354–369.
O’Neill C (1997) Economic Impact of Zebra Mussels: Results of the 1995 Zebra Mussel Information Clearinghouse Study. Great Lakes Research Review 3(1): 35–42
Offutt Air Force Base (2009) Final summary report: Zebra Mussel Eradication Project, Lake Offutt Nebraska. Omaha (NE): OAFB prepared for 55 CES/CEV, pp 1–51
Pacific Northwest Economic Region Foundation and Pacific States Marine Fisheries Commission (2015) Advancing a Regional Defense against Dreissenids in the Pacific Northwest. Report prepared by Creative Resource Strategies, LLC, Salem, OR, pp 1–32.
Sun JF (1994) The Evaluation of Impacts of Colonization of Zebra Mussels on the Recreational Demand in Lake Erie. In: Proceedings of the Fourth International Zebra Mussel Conference, (March 7-10, 1994), University of Wisconsin Sea Grant Institute, Madison, Wisconsin. pp 647–660
Watters A, Gerstenberger SL, Wong WH (2013) Effectiveness of EarthTec® for killing invasive quagga mussels (Dreissena rostriformis bugensis) and preventing their colonization in the Western United States. Biofouling: The Journal of Bioadhesion and Biofilm Research 29: 21–28.
Western Governors’ Association (2018) Top 50 Invasive Species in the West. Denver, CO 80202.
